
How Long Can A Pokemon Stay In A Gym, How long can a Pokemon stay in a gym without berries, 1.26 MB, 00:55, 640, Ask About SPORTS, 2020-08-07T21:37:34.000000Z, 19, How long can a pokemon stay on a gym? | Pokemon GO Wiki - GamePress, pokemongo.gamepress.gg, 1440 x 1280, png, pokemon gym stay gamepress pokemongo, 20, how-long-can-a-pokemon-stay-in-a-gym, KAMPION
What is the ph of a solution of mg (oh)2 after equilibrium is established? You need to perform the ice table analysis. Ph of 0. 32 m mg(oh) 2. Magnesium hydroxide (mg(oh) 2) is not asoluble hydroxide in water.
First, you should know that, is it. And thus, it supposedly gives rise to 0. 026 × 2 = 0. 052 m oh−. As a result, but clearly, we have the poh and not the ph. At any temperature, and at 25∘c, pkw = 14. But as chemists, we must check the data. The ksp of sr(oh)2 is around 6. 4 × 10−3. Visit byjus to study the uses, physical and chemical properties, structure of magnesium hydroxide (mg(oh)2) from the experts. At neutral ph levels and higher, magnesium hydroxide is nearly insoluble in water. Keeping this in mind, mag has little to no.
Calculate the pH at which Mg(OH)2 begin to precipitate from solution

CM4106 Review of Lesson 4
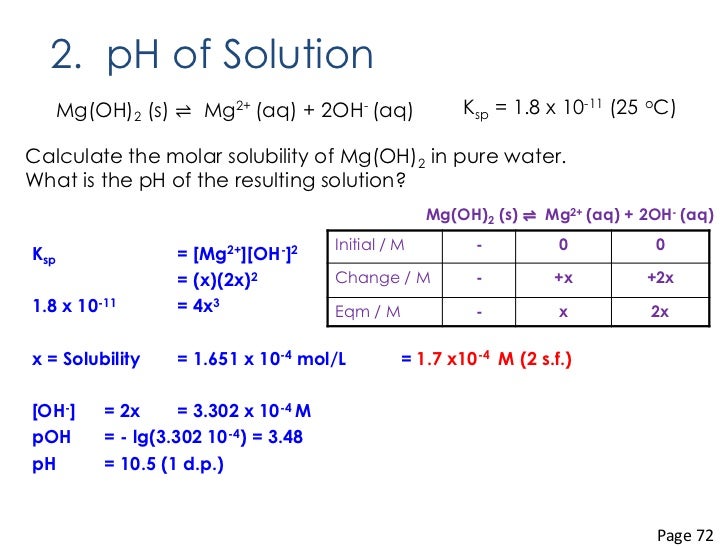
CM4106 Review of Lesson 4
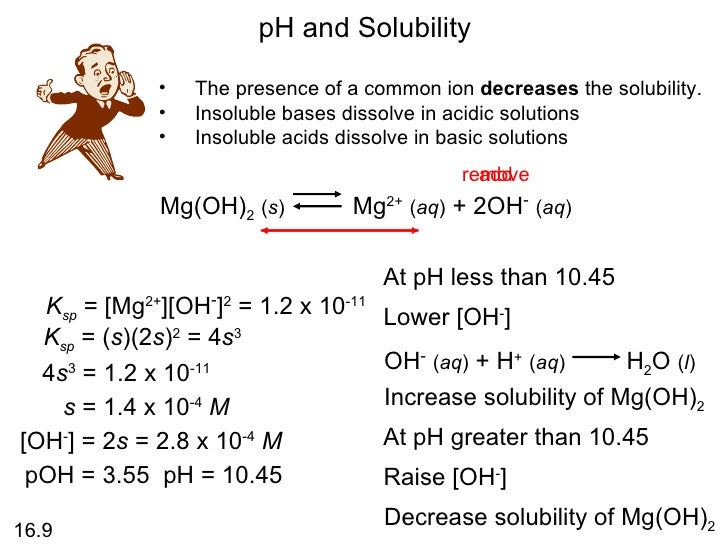
pH of AMD with Ca(OH) 2 , Mg(OH) 2 and CaMg.2(OH) 2 with mixing and

Chemistry Equilibrium
At `25^(@)C`, the solubility product of `Mg(OH)_(2)` is `1.0xx10^(-11

Calculate the pH at which Mg(OH)2 begin to precipitate from solution

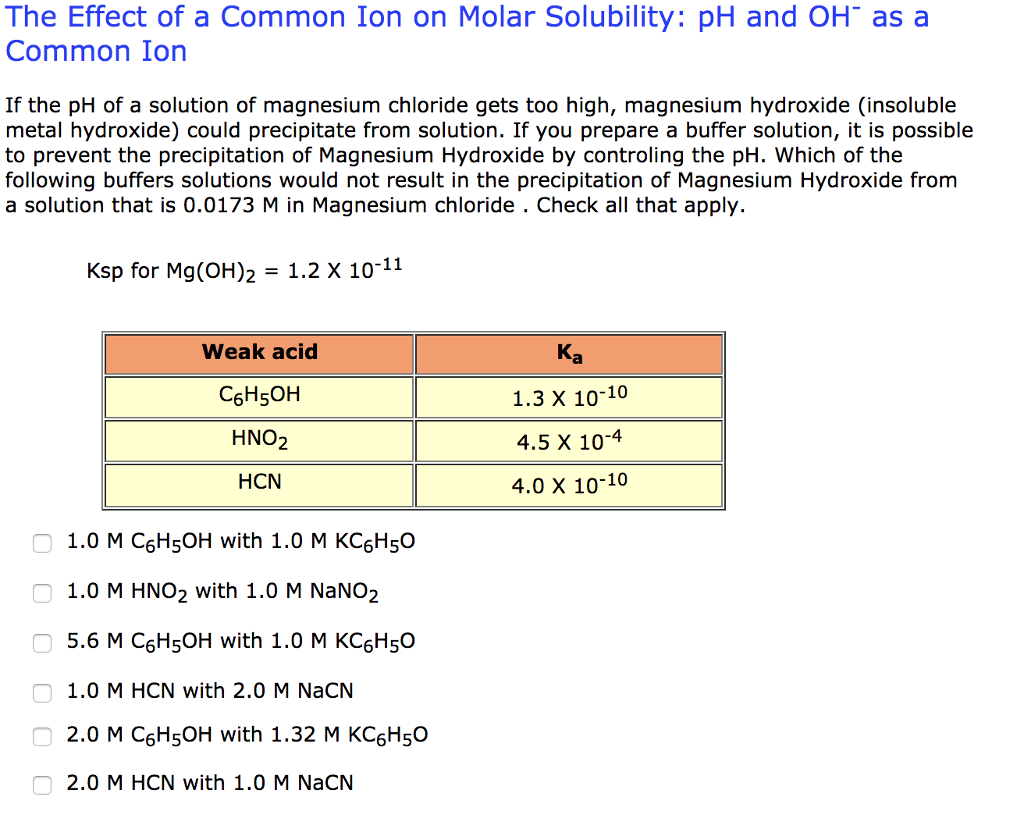
Solved: If The PH Of A Solution Of Magnesium Chloride Gets... | Chegg.com
Nilai Ksp Ca(OH)2 dan Mg(OH)2 adalah 6,5×10^-6 dan 7,1×10-12. pH
`K_(sp)` of `Mg(OH)_(2)` is `4.0 xx 10^(-6)`. At what minimum `pH, Mg

EmoticonEmoticon