How Long Can A Pokemon Stay In A Gym, How long can a Pokemon stay in a gym without berries, 1.26 MB, 00:55, 640, Ask About SPORTS, 2020-08-07T21:37:34.000000Z, 19, How long can a pokemon stay on a gym? | Pokemon GO Wiki - GamePress, pokemongo.gamepress.gg, 1440 x 1280, png, pokemon gym stay gamepress pokemongo, 20, how-long-can-a-pokemon-stay-in-a-gym, KAMPION
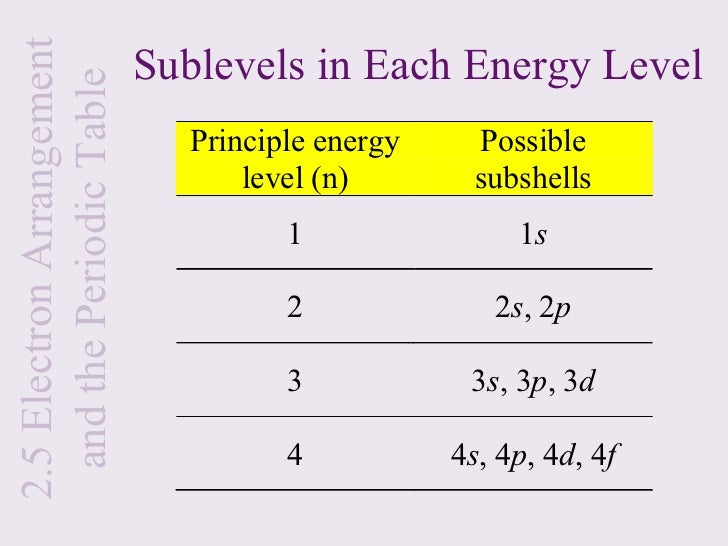
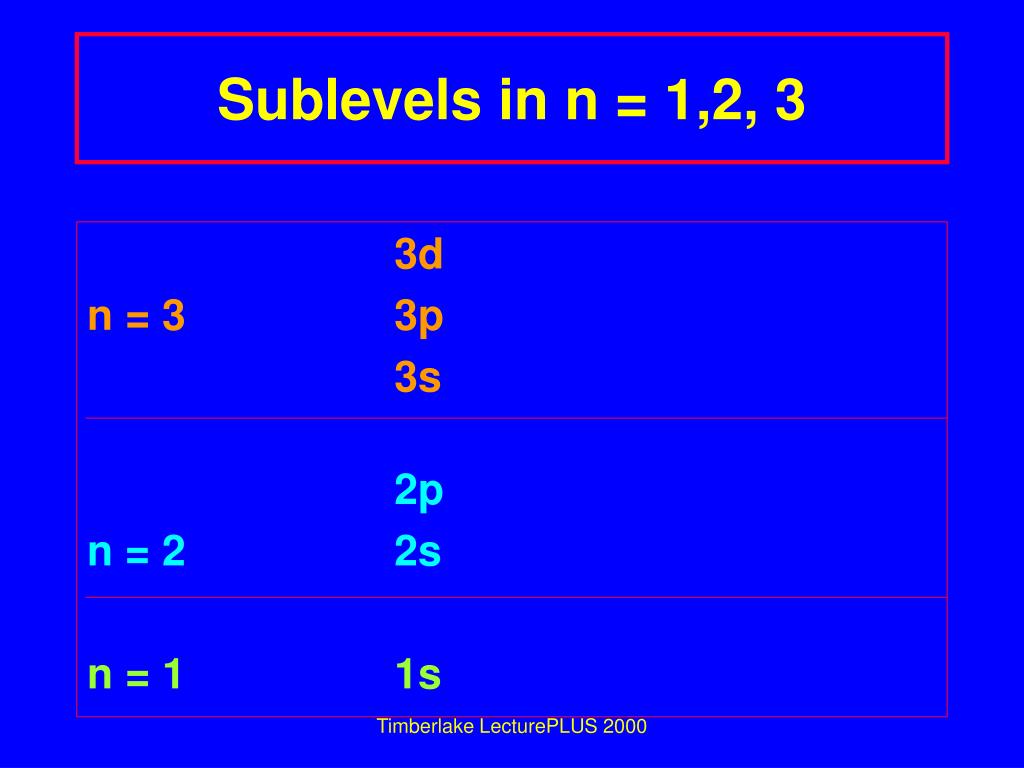
I have written a similar answer to this question, i will copy it here i hope it helps: Shell is the energy level of an atom ( n = 1,2,3,etc. Inside every shell there is one or more subshells ( s,p,d,f,etc. Inside every subshell there is one or more orbitals.
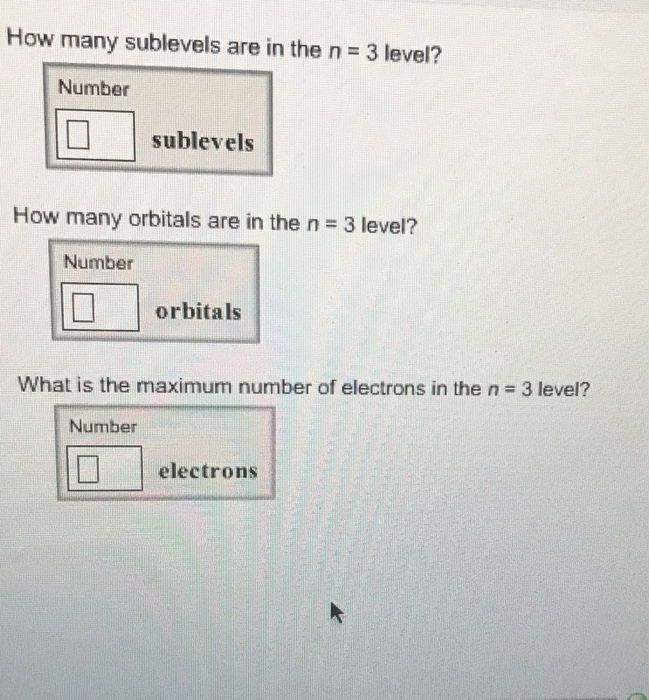
The p subshell contains 3 orbitals. Solution for how many sublevels are in the n = 3 level? How many orbitals are in the n = 3 level? What is the maximum number of electrons… If n (principal quantum number) = 3, then l (azimuthal quantum number) = 0, 1, 2 this means that there are three possible types of subshells available in the third energy level. Such subshells are s, p, and d. Use the formula 2n2. In this case n = 3, so 2 (32) = 18. The sublevels and number of electrons in each are 3s23p63d10, for a total of 18 electrons.
Mec chapter 2

How many orbitals are in the n = 3 level? - Brainly.com

Solved: 18) For N 3, What Are The Possible Sublevels? A) 0... | Chegg.com

PPT - Subshells and Orbitals PowerPoint Presentation - ID:457731
Solved: How Many Sublevels Are In The N 3 Level? Number Su... | Chegg.com
Solved: How Many Sublevels Are In The N 3 Level? Number Su... | Chegg.com
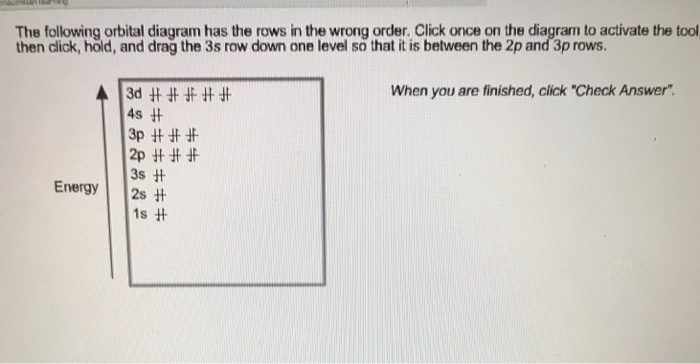
Electronic structure
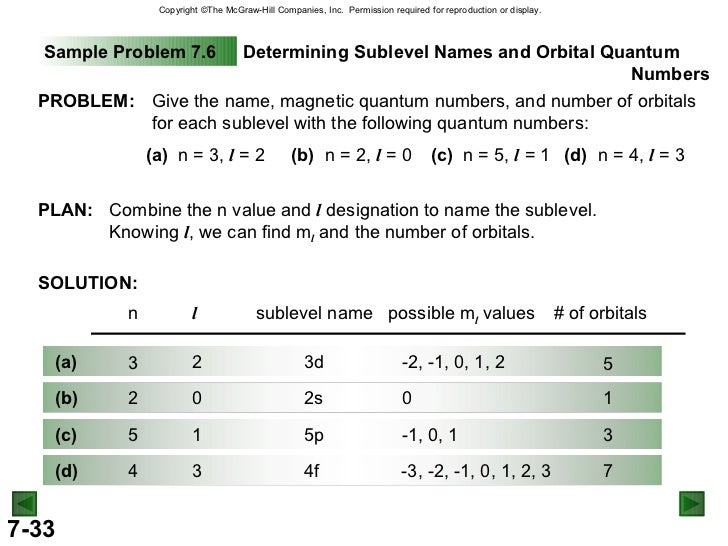
New chm 151_unit_4_power_points
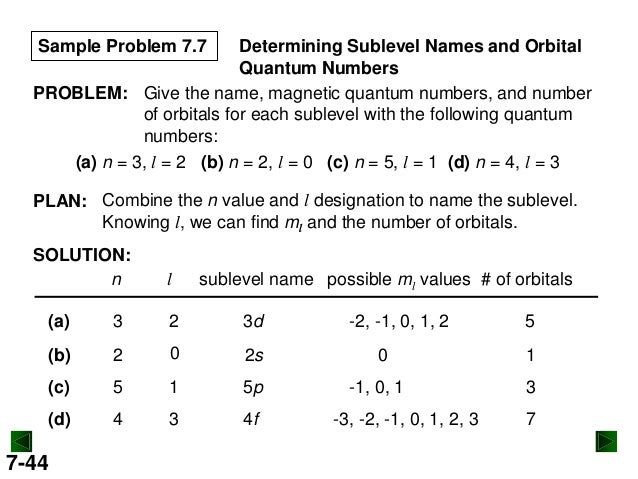
Solved: How Many Sublevels Are In The N = 3 Level? Subleve... | Chegg.com
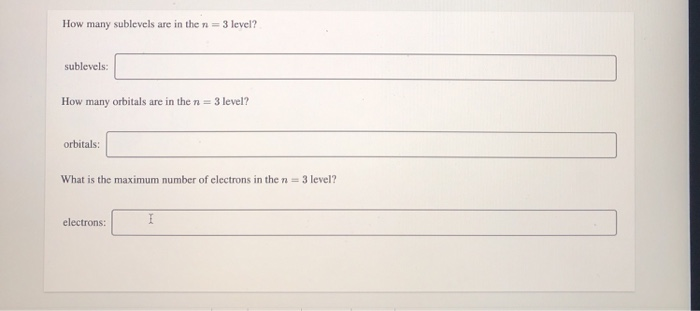
Solved: How Many Sublevels Are In The N 3 Level? Number Su... | Chegg.com

EmoticonEmoticon