How Long Can A Pokemon Stay In A Gym, How long can a Pokemon stay in a gym without berries, 1.26 MB, 00:55, 640, Ask About SPORTS, 2020-08-07T21:37:34.000000Z, 19, How long can a pokemon stay on a gym? | Pokemon GO Wiki - GamePress, pokemongo.gamepress.gg, 1440 x 1280, png, pokemon gym stay gamepress pokemongo, 20, how-long-can-a-pokemon-stay-in-a-gym, KAMPION
Both are soluble in water. It’s a matter of the degree of solubility based on the acidity of the ion. I lifted the rule from a web page with no proper author attribution but this is as i remember it: “the rule is z^2/r, where z is the charge and r is the radius of the atom.
Click on to see full reply supportmymoto Usually there is a difference between water soluble fe 2+ compounds and generally water insoluble fe 3+ compounds. Fe(oh)3 f e ( o h ) 3 is only slightly soluble in water which means only a very small amount will dissolve. Utilizing the solubility rules, most of the hydroxide salts (such as iron (iii) oxide) are slightly soluble in water. (ii) fe (oh)3is less soluble than fe (oh)2in water because fe3+is smaller than fe2+and thus charge is more. Therefore, fe (oh)3is more covalent than fe (oh)2. (iii) the colour of some compounds can be explained on the basis of polarisation of their bigger negative ions. For example:agcl is white agbr, ag , ag2co3are yellow. Feo (oh) ( iron (iii) hydroxide ) is insoluble in water.
Is Fe(OH)3 Soluble or Insoluble in Water? - YouTube
Equation for Fe(OH)3 + H2O | Iron (III) hydroxide + Water - YouTube
What is the molar solubility of fe(oh)3 in pure water? (ksp for fe(oh)3

inorganic chemistry - Is Fe(OH)2 soluble in alkalis? - Chemistry Stack

lecture
Ferric Ion (Fe3+) Can Form Various Complexes As Fo... | Chegg.com
Complex equilbrium
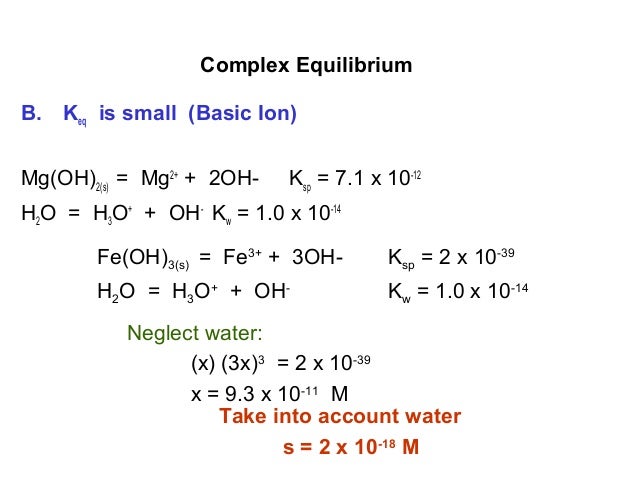
Aqueous Metal Ions
Solved: 6. Calculate The Values Of [Fe3+], PE, And PH At T... | Chegg.com
![Is Fe(oh)3 Soluble In Water Solved: 6. Calculate The Values Of [Fe3+], PE, And PH At T... | Chegg.com](https://media.cheggcdn.com/media/984/984f3470-8898-4574-980a-ae9cc5c338a5/phpmxTyf9.png)
EmoticonEmoticon